Coni-Snap Sigma Series Capsules
Marketplace Demands for Sigma Quality Levels
Quality by Design (QbD) initiatives have become a new priority in pharmaceutical manufacturing as companies are caught between heightened regulatory scrutiny and the demand for greater operational efficiency. The key principle of QbD is to build more robust and tighter process capabilities with reduced variation—to build the quality into the product and the processes in order to reduce reject rates.
Introducing a New Milestone in Capsule Quality and Value – Coni-Snap® Sigma Series Capsules
In 2013, Capsugel launched the first stage in a paradigm shift for quality in capsules with the introduction of Coni-Snap Sigma Series capsules. Sigma Series capsules is the designation given all capsules manufactured to sigma quality standards—with quality measured to a parts per million (PPM) level instead of the industry standard Acceptable Quality Levels (AQL) percentages. All Capsugel capsules—printed and unprinted—will be the product of this unparalleled increase in quality standards as this implementation schedule rolls out across all products and around the world through 2014 and 2015.
A Coni-Snap Capsule with Superior Visual and Print Quality, Produced with Advanced Manufacturing Technology
- Improved quality, safety & compliance
- Move from AQL sampling to PPM level controls & Sigma-level specifications
- Efficient implementation
- No filing/registration requirements expected
- No changes to encapsulation processes
- Potential reduction for patient complaints
 Produced on Re-Designed High Precision Manufacturing and Printing Lines
Produced on Re-Designed High Precision Manufacturing and Printing Lines
- Superior quality obtained through the combination of process technology and online electronic inspection of every single capsule
- Greater quality consistency within each lot, with substantially reduced defect levels
- New defect level specifications based on ppm levels
Coni-Snap Sigma Series Capsules – Improvement from Incoming Inspection to Consumer
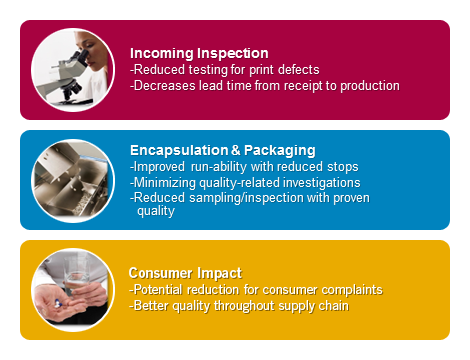
All along on the supply chain, Coni-Snap Sigma Series is designed to operate with greater assurance of quality, safety and regulatory compliance